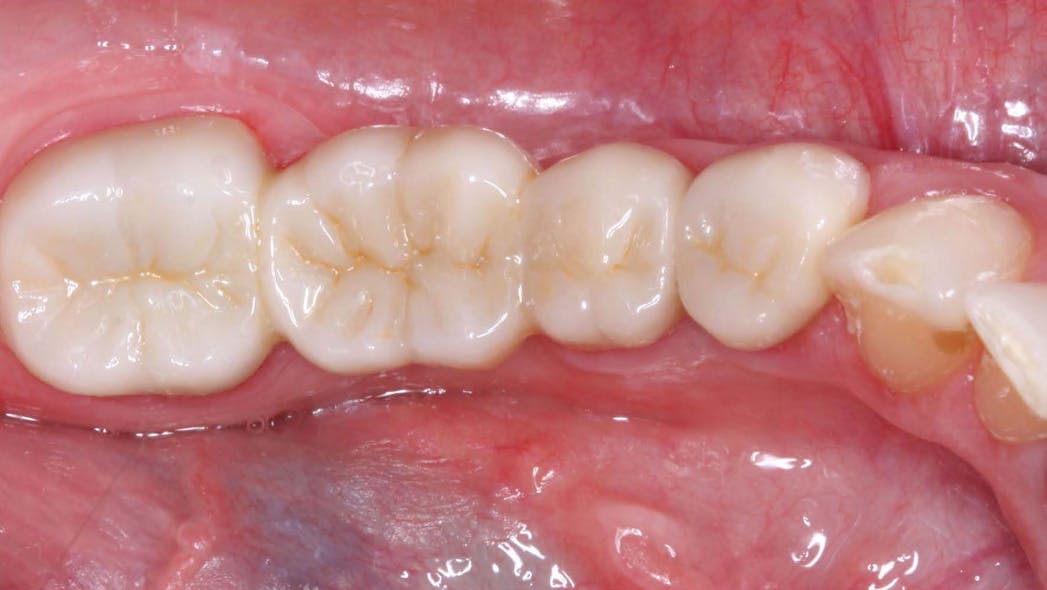
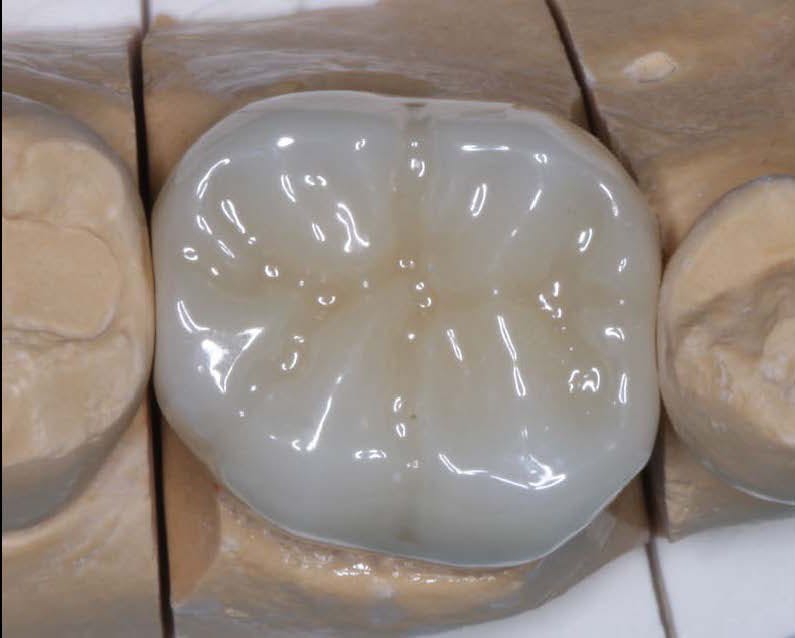
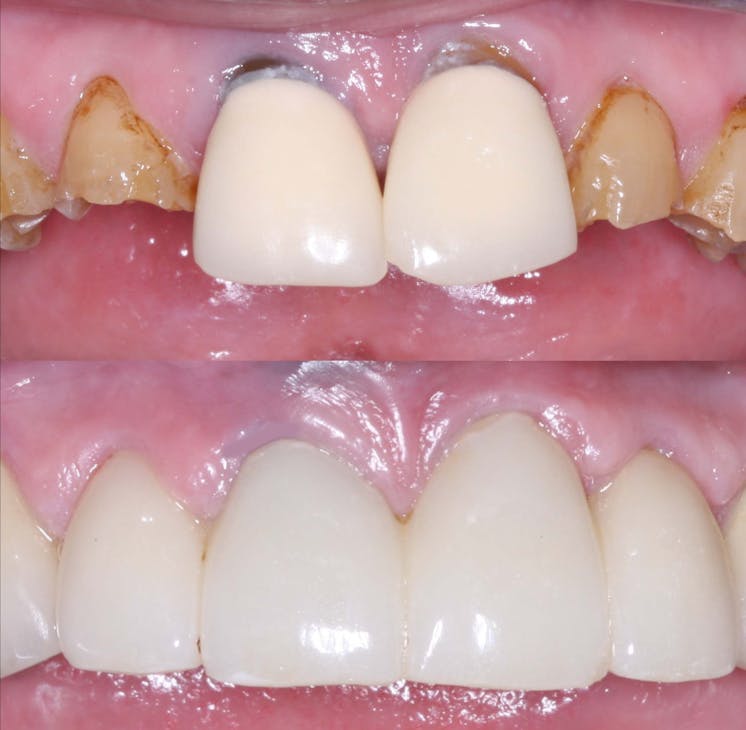
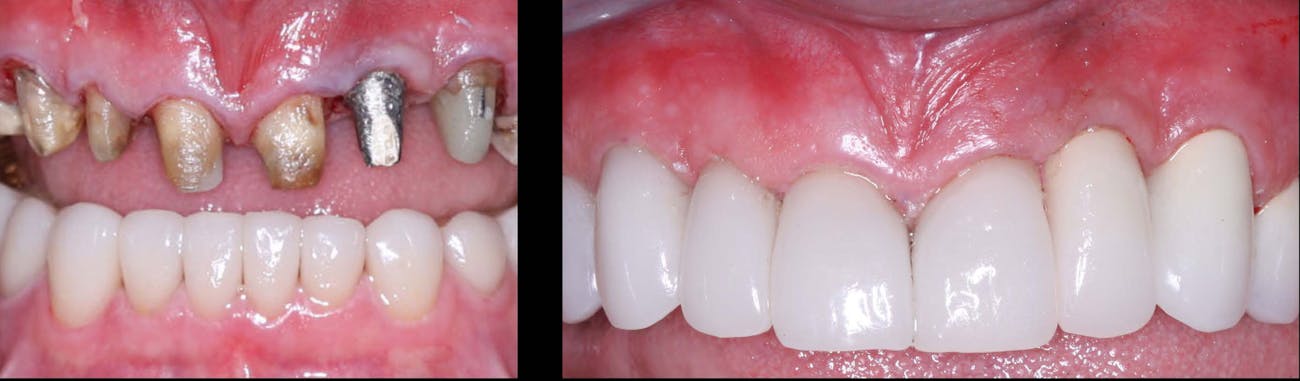
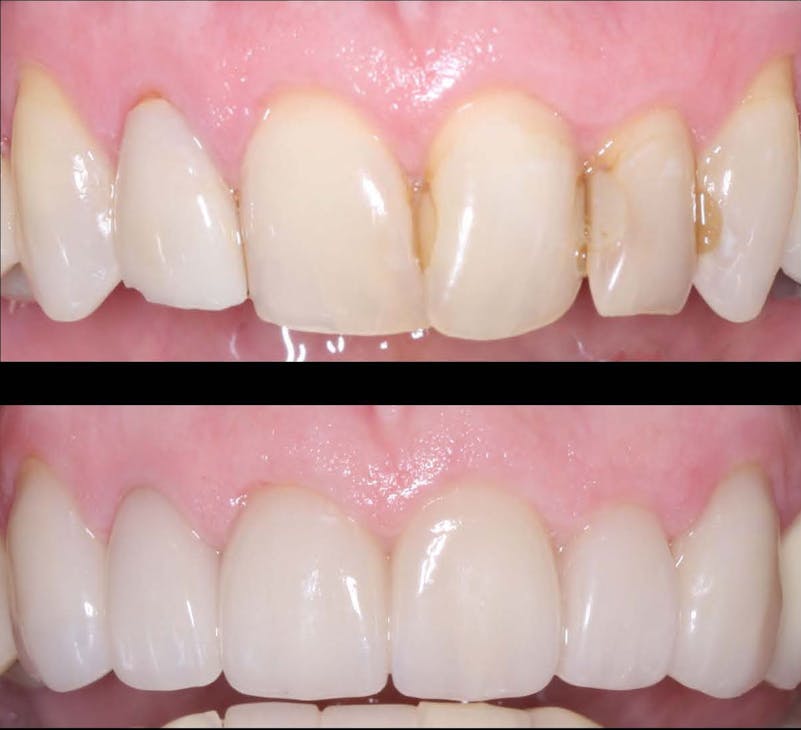
In the realm of dental crown placement, adhesive bonding techniques have undergone significant advancements, revolutionizing the way dental crowns are attached to natural teeth. These innovative bonding systems and techniques have propelled the field of dentistry forward, offering improved bond strength, enhanced durability, and simplified application methods. In this blog post, we will delve into the latest adhesive bonding techniques for dental crowns, showcasing the advancements in bond strength, durability, and ease of use, ultimately leading to successful and long-lasting restorations.
I. Understanding Adhesive Bonding in Dental Crown Placement:
A. Overview of adhesive bonding and its benefits
B. Importance of a strong bond between the crown and tooth structure
C. Evolution from traditional cementation to adhesive bonding
II. Latest Adhesive Systems for Dental Crown Bonding: A. Resin-based adhesive systems
- Introduction to self-etch and total-etch adhesive systems
- Advantages and considerations of different adhesive systems B. Bonding agents and primers
- Role of bonding agents in enhancing bond strength
- Advances in primers for reliable adhesion to different tooth substrates C. Dual-cure and light-cure materials
- Benefits of dual-cure materials in challenging clinical situations
- Advancements in light-cure materials for simplified application
III. Advancements in Adhesive Bonding Techniques:
A. Selective enamel etching and conditioning
- Techniques to preserve enamel and increase bond strength
- Advances in selective etching protocols B. Use of adhesive protocols with different substrate conditions
- Bonding techniques for vital and non-vital teeth
- Strategies for bonding to previously restored teeth or dental implants C. Proper isolation and moisture control
- Importance of a dry and isolated field for optimal bond strength
- Innovative isolation techniques and materials for effective moisture control
IV. Benefits and Considerations of Adhesive Bonding for Dental Crowns:
A. Improved bond strength and durability
- Enhanced retention and resistance to mechanical forces
- Reduced risk of microleakage and secondary caries B. Conservative tooth preparation and preservation
- Minimal tooth reduction for adhesive bonding compared to traditional methods
- Preservation of healthy tooth structure and long-term tooth health C. Simplified application and enhanced user experience
- User-friendly techniques for efficient bonding procedures
- Time-saving benefits and improved patient comfort
Conclusion: The advancements in adhesive bonding techniques for dental crowns have revolutionized the field of dentistry, offering superior bond strength, enhanced durability, and simplified application methods. With the latest resin-based adhesive systems, bonding agents, and improved techniques, dental professionals can achieve long-lasting and esthetically pleasing restorations while preserving healthy tooth structure. By embracing these advancements, dentists can deliver optimal outcomes, ensuring the success and satisfaction of both clinicians and patients in dental crown placements.










Recent Comments