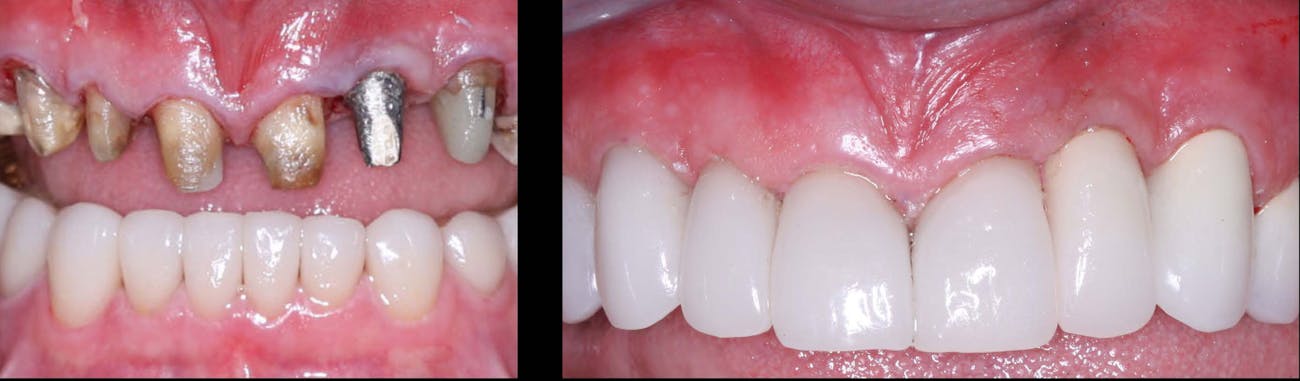
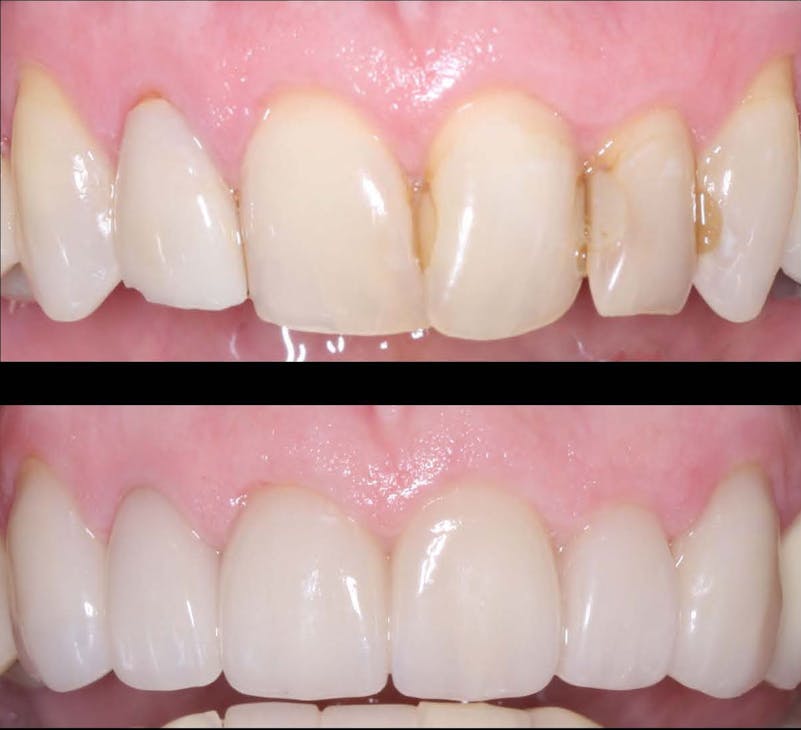
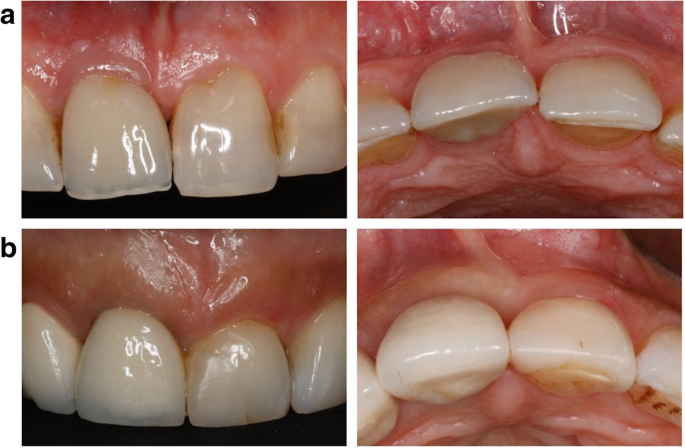
When it comes to restorative dentistry, zirconia crowns have emerged as a superior solution for patients seeking durable and esthetically pleasing dental restorations. Zirconia, a biocompatible ceramic material, offers a range of advantages that make it a popular choice among dentists and patients alike. In this blog post, we will delve into the benefits of zirconia crowns, exploring their strength, aesthetics, and long-term reliability. As a trusted dental lab specializing in zirconia crown fabrication, KC Dental Lab is committed to providing dentists with high-quality restorations that deliver exceptional outcomes.
I. Unparalleled Strength and Durability of Zirconia Crowns:
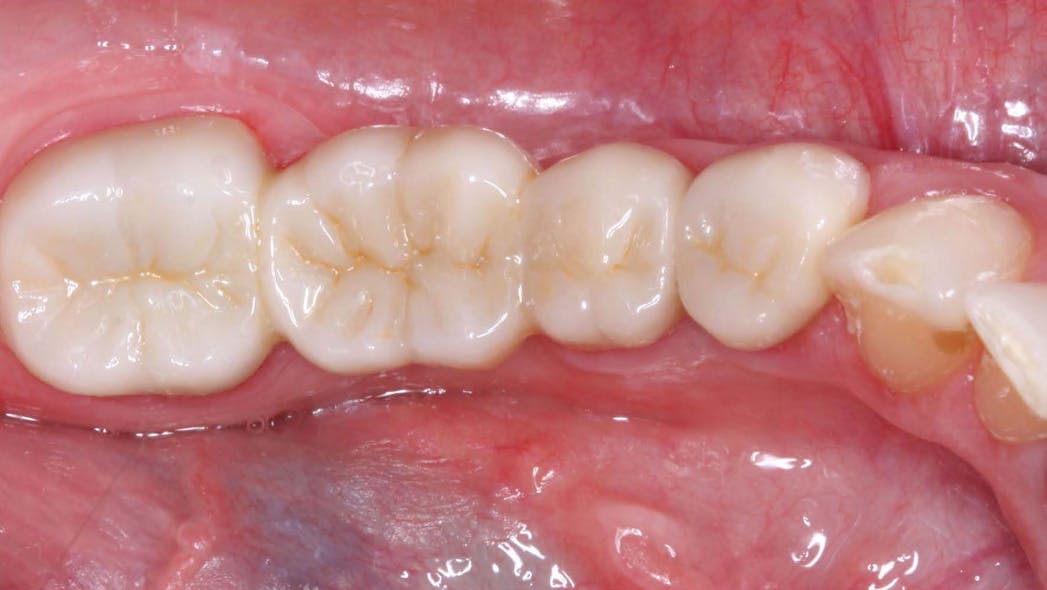
Zirconia crowns are renowned for their exceptional strength and durability, making them an ideal choice for both posterior and anterior restorations. The inherent properties of zirconia allow for excellent load-bearing capacity, resisting fractures and chipping. This durability ensures that zirconia crowns can withstand the forces of occlusion, providing long-lasting solutions for patients. By partnering with KC Dental Lab, dentists can offer their patients restorations that not only look natural but also offer unmatched strength and reliability.
II. Natural-Looking Aesthetics: Creating Beautiful Smiles:
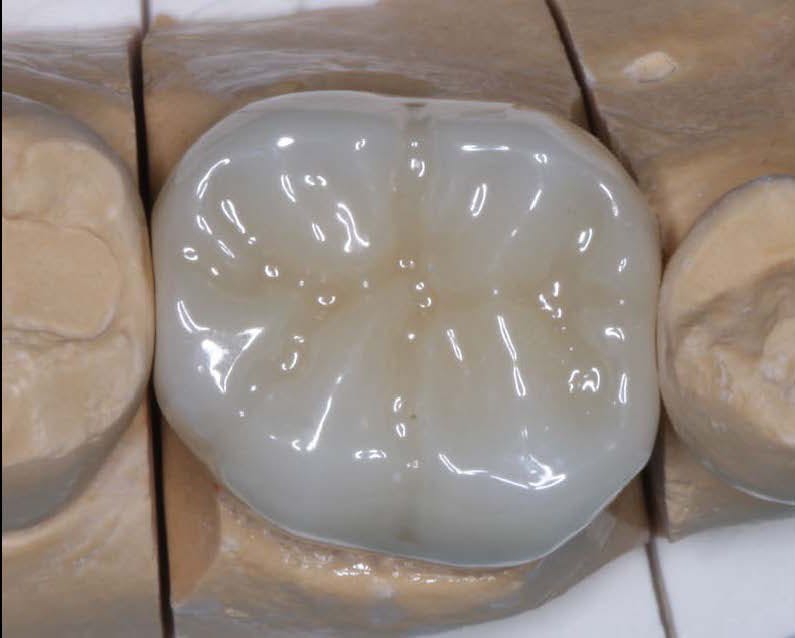
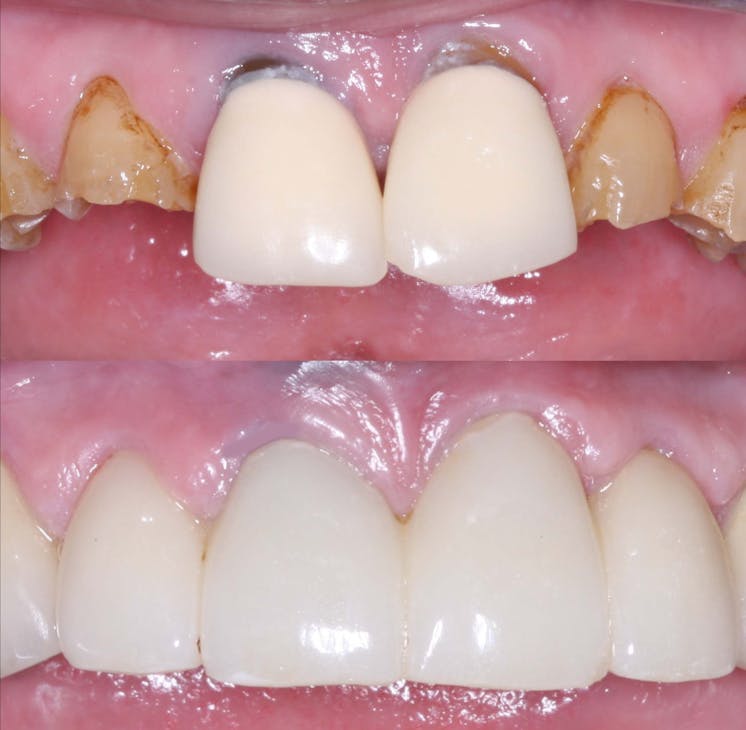
One of the key advantages of zirconia crowns is their ability to replicate the natural appearance of teeth. With their translucent properties and shade-matching capabilities, zirconia crowns blend seamlessly with the surrounding teeth, resulting in esthetically pleasing restorations. This makes zirconia crowns an excellent choice for patients who desire a beautiful smile. KC Dental Lab understands the importance of achieving natural-looking aesthetics and employs advanced techniques to create zirconia crowns that exhibit lifelike characteristics, enhancing patient satisfaction.
III. Biocompatibility and Oral Health Benefits:
Zirconia is a biocompatible material, meaning it is well-tolerated by the human body. Unlike certain metal-based restorations, zirconia crowns do not cause adverse reactions or allergic responses in patients. Additionally, zirconia crowns do not conduct heat or cold, minimizing sensitivity and discomfort for individuals with temperature sensitivity. Furthermore, zirconia crowns have a smooth surface that resists plaque accumulation, promoting better oral hygiene and reducing the risk of periodontal issues. By offering zirconia crowns fabricated by KC Dental Lab, dentists can ensure the oral health and well-being of their patients.
IV. Preserving Tooth Structure: Minimally Invasive Dentistry:
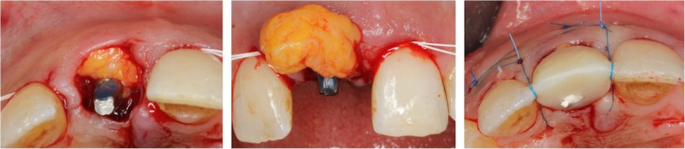
Another significant advantage of zirconia crowns is their ability to preserve natural tooth structure. Due to the high strength of zirconia, minimal reduction of the tooth is required for crown preparation. This conservative approach helps to maintain the integrity of the remaining tooth structure, reducing the need for extensive drilling and preserving healthy tooth enamel. With zirconia crowns, dentists can provide their patients with minimally invasive dentistry, promoting long-term oral health.
V. Long-Term Reliability and Patient Satisfaction:
Zirconia crowns have a proven track record of long-term success, offering patients reliable restorations that stand the test of time. With proper oral hygiene and regular dental visits, zirconia crowns can last for many years, providing patients with a durable and functional solution. The combination of strength, aesthetics, biocompatibility, and preservation of tooth structure contributes to high patient satisfaction. By partnering with KC Dental Lab for zirconia crown fabrication, dentists can ensure that their patients receive restorations that not only meet their functional needs but also exceed their expectations in terms of aesthetics and longevity.


Recent Comments